What is Zeta Potential?
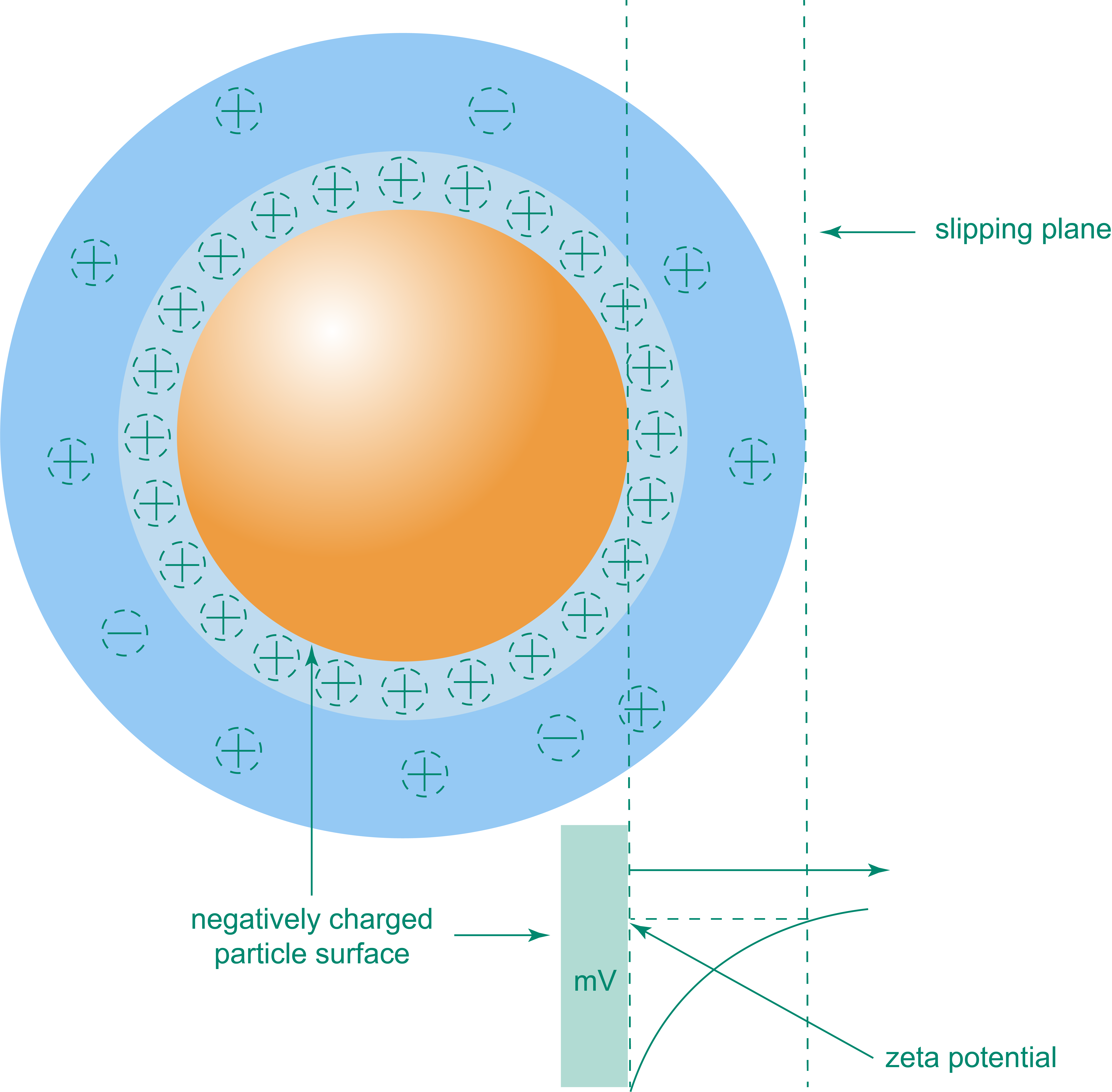
Zeta potential (ZP) is a physical property exhibited by any particle in a suspension, macromolecule or material surface. ZP is an analytical technique used to determine the surface charge of nanoparticles in colloidal solutions. The surface of a charged particle attracts and binds firmly to a thin layer of opposite charge, forming a thin layer of liquid called the Stern layer; as the particle diffuses in the solution, it will be replaced by a thin layer of loosely bound ions. The outer diffusion layer is involved, resulting in an electric double layer (Figure 1). Zp is named after the potential of the electric double layer and is determined by measuring the speed of charged particles moving through the sample solution toward the electrode in the presence of an external electric field. ZP values are typically in the range of +100 to −100 mV. The magnitude of the ZP gives a prediction of colloidal stability. The ZP of NPs with values >+25 mV or <−25 mV is generally highly stable. Lower dispersion ZP values can lead to aggregation, coagulation or flocculation due to van der Waals attraction between particles. Zeta potential can be used to optimize the formulation of suspensions, emulsions and protein solutions, predict interactions with surfaces, and optimize the formation of films and coatings. Understanding zeta potential can reduce the time required to produce pilot formulations. It can also be used as an aid in forecasting long-term stability.
Factors Affecting Zeta Potential
The most important factor affecting the zeta potential is the pH value of the medium. Other factors include ionic strength, concentration of any additives, and temperature.
- pH
The pH level can have a significant impact on the potential value of zeta potential. If a material’s surface chemistry has polar functional groups, then pH is the most critical factor affecting zeta potential. Due to the effect of pH on zeta potential results, all zeta potential results should correspond to the pH of the sample during analysis. Zeta potential without correlating pH is effectively meaningless. When diluting a sample for zeta potential analysis, it is important to maintain the pH at which the sample was pre-diluted so as not to affect the zeta potential of the suspension.
- Ionic strength
The conductivity of a solution is related to the free ions in the solution. The concentration of available ions forming a double layer around the particle will affect the potential at the slip plane, and the potential at the slip plane directly affects the Zeta potential value. The pH of the solution has a greater impact on the zeta potential, but the ionic strength also plays a role in the overall zeta potential.
- Temperature
Zeta potential is affected by temperature because both particle mobility and viscosity are affected by temperature changes.
Zeta Potential Measurement
Although the zeta potential cannot be measured directly, it can be calculated using theoretical models. Electrokinetic phenomena and electroacoustic phenomena are common sources of data for calculating zeta potential. The zeta potential of a dispersion is measured by applying an electric field across the dispersion. Particles with a zeta potential in the dispersion will migrate toward the oppositely charged electrode at a rate proportional to the magnitude of the zeta potential. There are two different experimental techniques: microelectrophoresis, which has the advantage of producing images of moving particles, and electrophoretic light scattering, which is based on dynamic light scattering. The latter can be used to characterize very small particles. The frequency shift or phase shift of the incident laser beam caused by these moving particles is measured as the mobility of the particles, and this mobility is converted into the Zeta potential using Smoluchowski or Huckel theory.
Significance of Zeta Potential
- Pharmaceutical industry
Nanoparticle surface is a very important factor in targeted drug delivery. Indeed, once in the bloodstream, conventional nanoparticles (no surface medication) and negatively charged particles can be rapidly opsonized and cleared in large quantities by fixed macrophages. Surface modification of these polymeric nanoparticle systems with hydrophilic polymers is the most common method to control the opsonization process and improve the surface properties of the system, especially the surface charge. Zeta potential, determined by surface charge, is very important for the stability of nanoparticle suspensions and is a major factor in the initial adsorption of nanoparticles to cell membranes. After adsorption, the endocytic uptake rate depends on particle size. Zeta potential and size therefore influence the toxicity of nanoparticles.
- Cosmetics industry
Emulsions are one of the most commonly used vehicles in skin care because of their strong affinity to the skin and the variety of formula benefits they can provide. However, emulsions are thermodynamically unstable systems. Zeta potential can be used to predict emulsion instability and control the behavior of colloidal suspensions/emulsions.
- Environmental science
Suspended solids are common impurities in industrial and mining wastewater. Safety and aesthetics require that all water be essentially free of suspended solids. To meet water clarity requirements for reuse or discharge, suspended solids are often allowed to settle to the bottom or cream to the top of large tanks. The duration of these processes is strongly related to particle size, e.g. larger particles settle/cream faster. Therefore, if the particles can be made to agglomerate, the sedimentation process will be shorter and therefore the treatment operation will be faster and therefore cheaper. Additives can reduce the magnitude of the particle surface charge (zeta potential) to zero. This increases the number of particle collisions, thereby increasing the rate of flocculation. Therefore, zeta potential measurement can be used to monitor the effect of coagulant addition. The results are then used to optimize the treatment process.
Accessing Highly Stable Particles
CD Bioparticles offers a range of basic silica particles with terminal hydroxyl groups which give silica colloids a large negative zeta potential at neutral and basic pH. We also have plain fluorescent polystyrene particles with positive/negative/neutral zeta potential for your research.
Reference
Rasmussen, M. K., Pedersen, J. N., & Marie, R. (2020). Size and surface charge characterization of nanoparticles with a salt gradient. Nature communications, 11(1), 2337.
























